Our Discovery & Delivery Platforms
Revolutionary technologies that unlock novel therapeutics and ensure targeted delivery
Versatile Peptide Discovery Platform
An unbiased, ultra-versatile system that unlocks novel multi-cyclic peptides and small molecules, built upon 35+ years of continuous innovation.
Platform Advantages
Detailed Technologies & Research Breakthroughs
Pioneering methodologies that have shaped the landscape of peptide discovery and screening
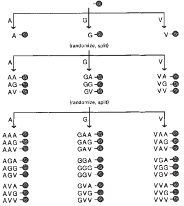
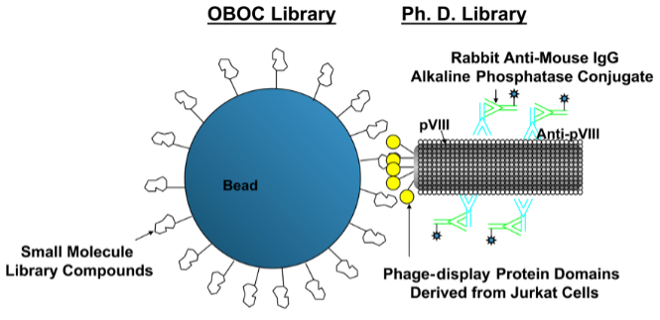
Pioneered the OBOC Technology
protein-binding ligand discovery (Lam et al.).
Nature 354 (6348):82-84

Cell Binding/Targeting Assays
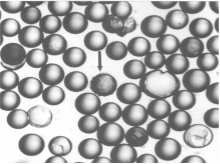
This technology isolates peptides binding to live cell surface receptors, enabling screening of conformationally sensitive receptors and selecting specific interactions with function-blocking antibodies.
DU-H prostate carcinoma cell attachment to peptide beads
Mol Divers 2 (1-2):19-28.
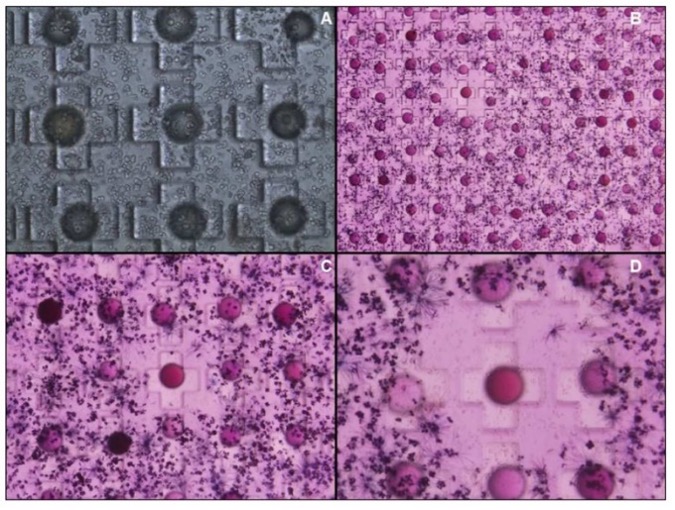
High-Throughput Screening with Microwell Technology
A powerful system for ultra-high-throughput, in situ, releasable solution-phase cell-based screening of functional molecules.
Townsend, J Comb Chem 12 (5):700-712. In-situ release of surface bound compound from dipeptide-based peptidomimetic library and resulting MTT cell viability assay.
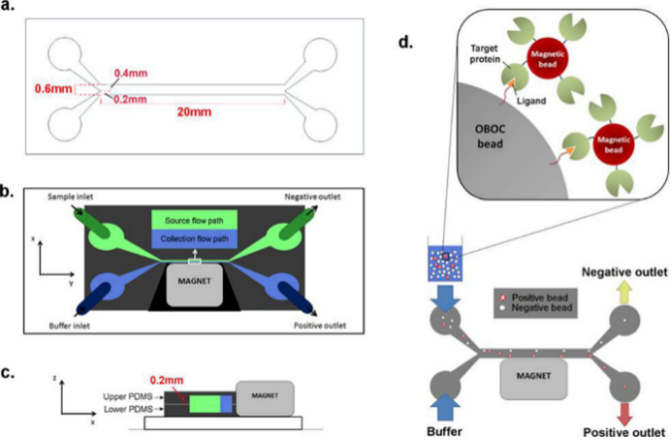
High-Throughput Screening with Microfluidics/Magnetics
This powerful technology presents a simple, cost-effective, and reliable automated platform for high-throughput magnetic sorting of large particles.
Cho et al., Price et al. ACS Comb Sci 18 (6):271-278.
Sophisticated One-Bead-Two-Compound Combinatorial Library
Rapidly discovers molecules that modulate cell signaling by screening live cells on bead surfaces. Demonstrated in discovering 'death ligands' for lymphoma cells.
Kumaresan PR, ACS Comb Sci 13 (3):259-264
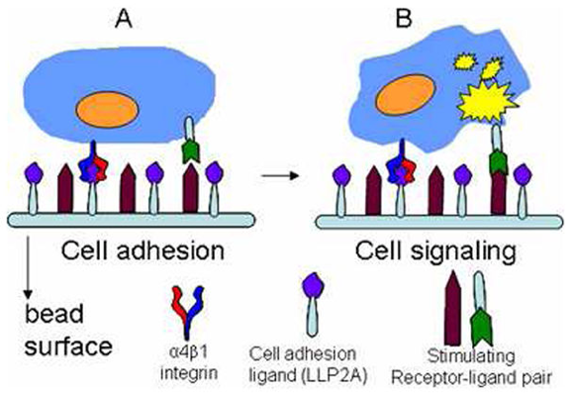
Multiplex Library-Against-Library Screening
Rapid discovery of functional molecular ligands (small molecule or peptides) against proteomic targets
Wu CY, ACS Comb Sci 18 (6):320-329
Precision Nano Delivery Platform
Stimuli-responsive, tumor-targeting nanocarriers engineered for controlled, on-demand therapeutic delivery with enhanced efficacy and minimized toxicity.
With 15 years of dedicated research and validated clinical trials, our advanced drug delivery technologies aim to improve therapeutic efficacy and minimize off-target effects.
Platform Technologies
Precision Targeting Delivery System (Nanomicelles)
Engineered to target cell surface receptors on cancer cells, these nano-carriers provide multi-specific, accurate intracellular delivery, and enhanced precision of cancer treatment.
Tunable Nanocarrier Systems
These systems have customizable features, high loading capacity, and enhanced stability for hydrophobic anticancer agents, utilizing the EPR effect for targeted tumor delivery.
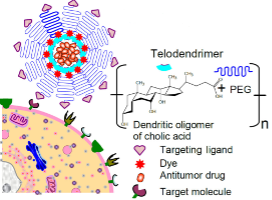
Peptide-Drug Conjugate (PDC) Platform
This platform combines peptide ligands for specific tumor binding with powerful drug payloads, effectively inhibiting tumor growth while reducing systemic side effects.
Advanced Delivery Technologies
Cutting-edge nanomedicine approaches for precise therapeutic delivery and enhanced treatment efficacy
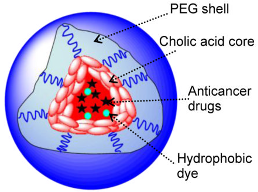
Versatile Stimuli-Responsive Nanocarrier Systems
Versatile Stimuli-Responsive Nanocarrier systems offer tunable properties, superior loading, and stability for hydrophobic anticancer drugs, enabling targeted tumor delivery via EPR effect, leading to improved efficacy and potentially less toxicity than conventional formulations.
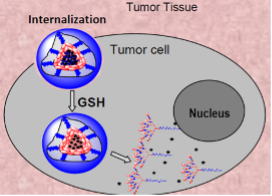
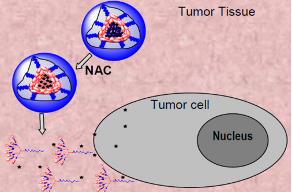
Glutathione-Responsive Drug Release
The high concentration of glutathione (GSH) found inside tumor cells can cleave the disulfide crosslinks in accumulated Paclitaxel-loaded disulfide crosslinked micelles. This cleavage causes the micelles to disintegrate, leading to the release of the encapsulated drug within the cells.
N-acetylcysteine Triggered Release
N-acetylcysteine (NAC), which is FDA-approved, can also cleave the disulfide crosslinks of accumulated Paclitaxel-loaded disulfide crosslinked micelles. This mechanism allows for on-demand drug release at the tumor site, potentially triggered by external administration of NAC.
Precision Targeting Delivery System
A tumor-targeting nanomicelle system precisely deliver drug payload into cancer cells by binding to the CLL1 molecule on their surface. Approximately 13.5-15 nm in size, these nanomicelles achieve intracellular delivery, including to the nucleus, offering potential to overcome chemoresistance and enhance targeted cancer treatment.
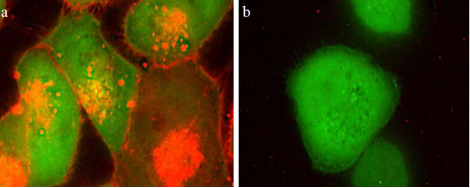
CLL1-Targeted Nanomicelles Validation
A549 cells expressing GFP-CLL1 (panel a) or GFP (panel b) were incubated with targeting nanomicelles loaded with DiI for 15 minutes before washing. The targeting ligand was identified from the Peptide Discovery Platform.
Hongyong Zhang, JNanomedicine. 2012 October ; 8(7): 1116–1124.
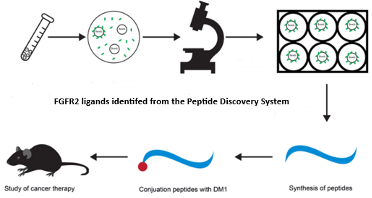
Peptide-Drug Conjugate Platform
Leveraging identified peptide ligands for selective binding to FGFR2-positive tumors, this peptide-drug conjugate (PDC) delivers the potent cytotoxin DM1 directly to cancer cells. This targeted approach potently inhibits tumor growth while significantly mitigating DM1's systemic side effects.
Wang, Y.; Biomedicines 2021, 9, 849.
Ready to Transform Medicine Together?
Our cutting-edge platforms are designed to accelerate drug discovery and delivery. Join us in creating the next generation of precision therapeutics.