Preclinical Drug Development CRO/CDMO Services
Providing integrated solutions for:
- Nanobody Discovery
- Peptide Discovery
- Nano Delivery Platform
- ADC payload solutions
- Small molecule chemistry
- CDMO/GMP manufacturing
- ADME/DMPK
- Oncology models
Solutions
Professional preclinical R&D solutions to accelerate your drug development
Nanobody Discovery
rapid discovery platform, 160+ validated targets, proprietary llama farm
- rapid discovery workflow
- 160+ validated target experience
- 12-acre llama farm
- Multiple display platforms (phage/yeast)
Peptide Discovery
Advanced peptide synthesis and screening platform for therapeutic peptide development
- Custom peptide synthesis
- High-throughput screening
- Structure-activity relationship studies
- Therapeutic peptide optimization
Nano Delivery Platform
Advanced nanoparticle drug delivery systems for enhanced bioavailability and targeted therapy
- Liposomal formulations
- Polymer nanoparticles
- Targeted drug delivery
- Bioavailability enhancement
ADC Payload Solutions
High-potency cytotoxic payloads and linker technologies for antibody-drug conjugates
- Cytotoxic payload synthesis
- Linker chemistry development
- ADC conjugation optimization
- Stability and efficacy testing
Small Molecule Chemistry
Custom synthesis from mg to kg scale, 100+ professional chemists, rapid delivery
- Custom synthesis: mg to kg scale
- Medicinal chemistry optimization
- Process development & scale-up
- Team led by former Merck scientists
CDMO/GMP Manufacturing
FDA/EU/China GMP compliant, 50L-10,000L production capacity
- Process development & optimization
- GMP manufacturing (multi-country certified)
- API production
- 50L-10,000L reactors
ADME/DMPK
Comprehensive in vitro and in vivo PK assessment, supporting rapid screening and optimization of drug candidates
- In vitro ADME (solubility, permeability, metabolism)
- In vivo DMPK (PK/PD studies)
- Multiple animal models
- Rapid professional reports
Oncology Models
CDX/PDX/Syngeneic models, professional in vivo efficacy assessment, accelerating anti-tumor drug development
- Multiple mouse models (CDX/PDX/Syngeneic)
- In vivo efficacy assessment
- Biomarker analysis
- Novopath proprietary platform
Consulting & Integrated Solutions
End-to-end drug development consulting, regulatory affairs, and integrated project management
- Regulatory filing preparation
- Integrated project management
- Drug development strategy
- Quality assurance & compliance
Why Choose Key2Meds
Faster
Accelerate project timelines
Rapid milestone delivery
Reliable
High-quality data support
For funding and regulatory submissions
Cost-Effective
Flexible collaboration models
FTE/FFS on-demand options
Comprehensive
One-stop solutions
From discovery to preclinical
Our Strength
Core Technology Platforms
Unique differentiated technology advantages
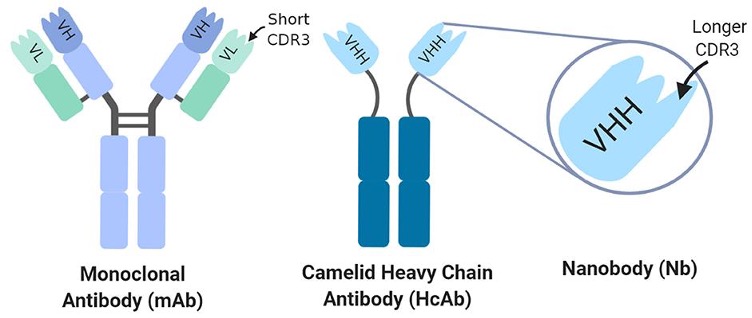
Nanobody Platform
- 12-acre proprietary llama farm
- Multiple display platforms (phage/yeast/rapid discovery)
- 17-24 weeks to selected Nanobodies
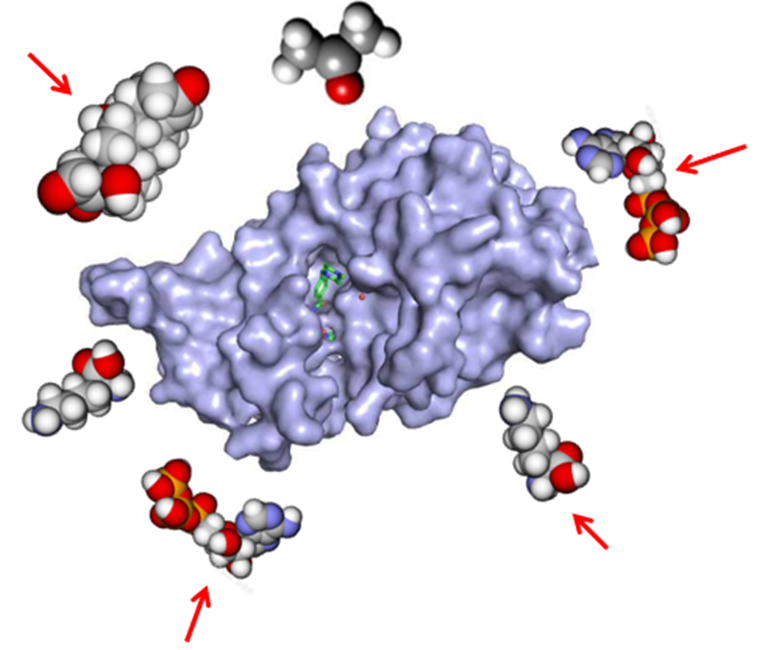
Peptide Platform
- Millions of unique peptide libraries
- Diversified screening and intelligent optimization
- Widely applied to targeting ligands and linkers
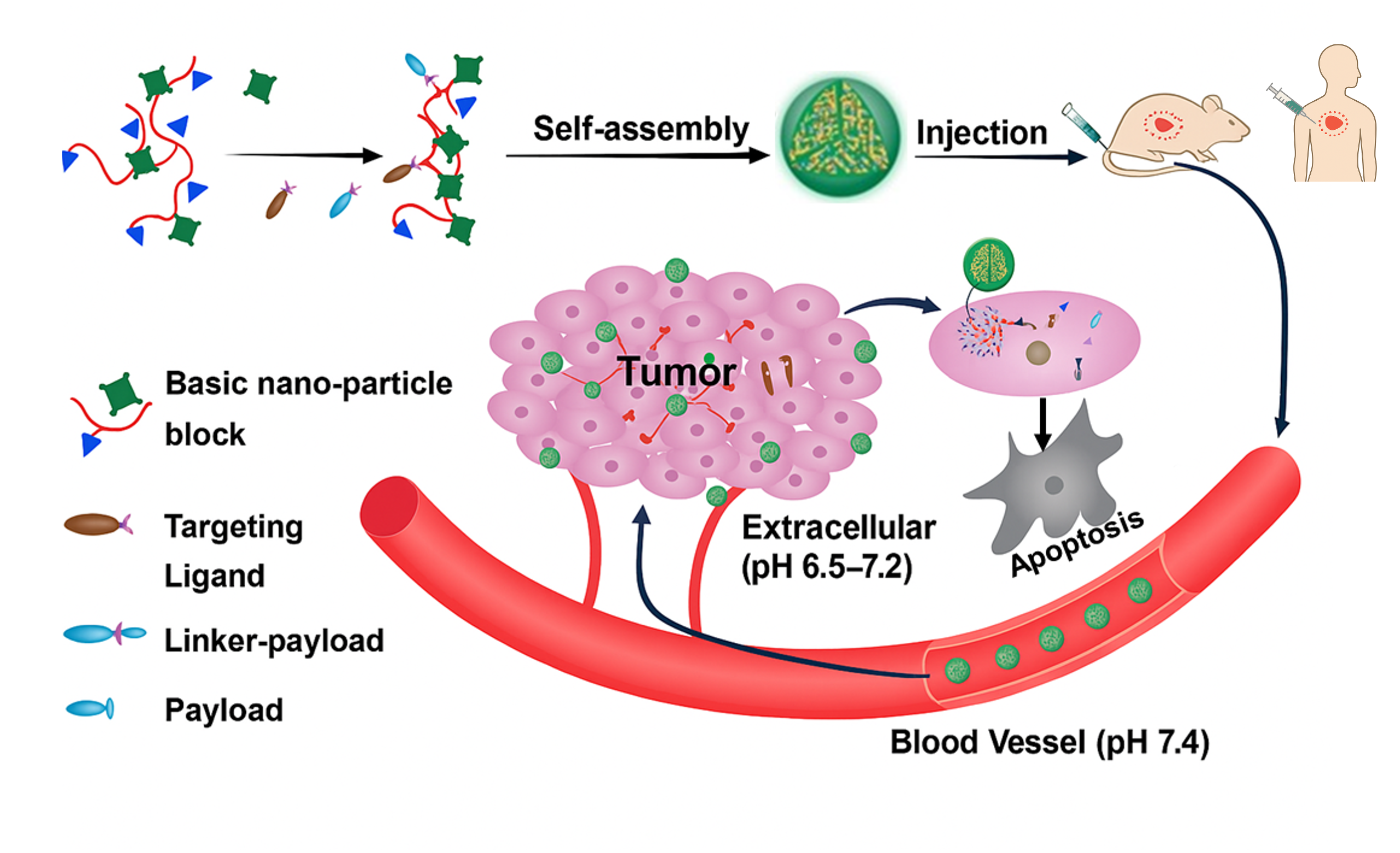
Nano Delivery Platform
- Liposomal formulations
- Polymer nanoparticles
- Targeted drug delivery
- Bioavailability enhancement
Service Process
Five-step process for seamless collaboration
Consultation
Free project assessment
Solution Design
Customized R&D plan
Project Execution
Expert team implementation
Quality Delivery
Data & report delivery
Ongoing Support
Follow-up optimization
Our Certifications
Passed 600+ government and client audits, compliant with global regulatory standards
Client Testimonials
Hear from our partners
"Key2Meds' nanobody platform helped us complete critical target validation in 3 months. The high-quality data supported our successful Series A funding."
"Their chemistry team is highly professional. The scale-up from mg to kg went smoothly with strict quality control and on-time delivery."
"CDMO services are FDA-compliant and helped us pass regulatory review smoothly. The project management team communicates promptly and responds quickly."
Ready to Start Your Drug Development Project?
Our expert team is ready to provide customized solutions
Accelerate your drug discovery and development process
Or contact us via:
Email: shawnmu02@yahoo.com
Business Hours: Monday-Friday 9:00-18:00 (Beijing Time)